COPD
Understanding COPD with a foundation of respiratory physiology thats needed to really get into it
Definition
- Chronic progressive obstructive respiratory disease characterized by airflow limitation that is not fully reversible
- Typically, but not universally, associated with long term smoking history
- Defined by a value of <0.7 FEV1/FVC
There are approx. 3 million COPD patient in the Uk and NICE estimates that there may even be 2 million more undiagnosed
Physiology Refresher - there is more depth later, this is just the intro
- So the function of the lung is to provide a large surface area in which to allow gas exchange into capillaries
- To do this the blood needs to meet the frequently refreshed air
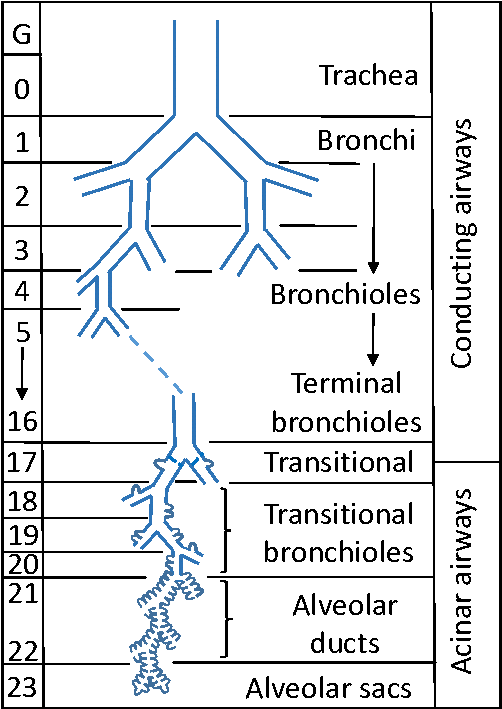
- The anatomy of the airways can be split into the Conducting Zone, the Transitional Zone and the Respiratory Zones
o The conduction zone starts at the trachea that bifurcates left and right and continues through 16 generations of splits through the Bronchi to the Terminal Bronchi
o Initially the airways have a lot of cartilage, but as you progress, this is replaced by smooth muscle
o The Conduction Zone has almost no gas exchange and so is referred to as anatomical dead space, takes up about 150ml of air
o The Transitional and Respiratory Zones start as the Terminal Bronchioles divide into Respiratory Bronchioles. This is where gas exchange can begin with occasional alveolar. The subdivision continues into Alveolar Ducts, lined with Alveoli, across a further 7 generations of division.
§ The area terminal to the Terminal Bronchiole is an anatomical unit called an Acinus
§ The distance from the terminal Bronchiole to the most distal alveoli may only be a few millimeters but accounts for up to 2.5-3L of lung volume
So how do we breathe?
Its all pressure gradients
- During inspiration the diaphragm contracts and descends the intercoastal muscles contract raising the ribs, this increases thoracic volume, reducing pressure
o This draws air in through any holes
§ Ideally this is through the upper airways and trachea
§ But it can be through any other hole (ie sucking stab wound, oesophageal rupture, any broncho-? fistulas)
§ The reducing pressure can be visualized clinically by watching the paradoxical movement of a fail segment (ie the fail moves in with inspiration rather than expanding out with the rest of the ribs)
o The air rushes in at a rapid rate in the upper airways but due to the ever increasing volume the pressure gradient is dispersed across the lungs and by the time the air movement reaches the alveoli its velocity is fairly low
§ This is important when we are ventilating patients and we need to think about baro and volume trauma
- Gas exchange is a continuous process that happens rapidly due to the short diffusion distance and large surface area, there is little concentration gradient in the Acinus due to this
The lung is elastic so much of expiration is passive contraction of the tissues as the intercostals and diaphragm relax
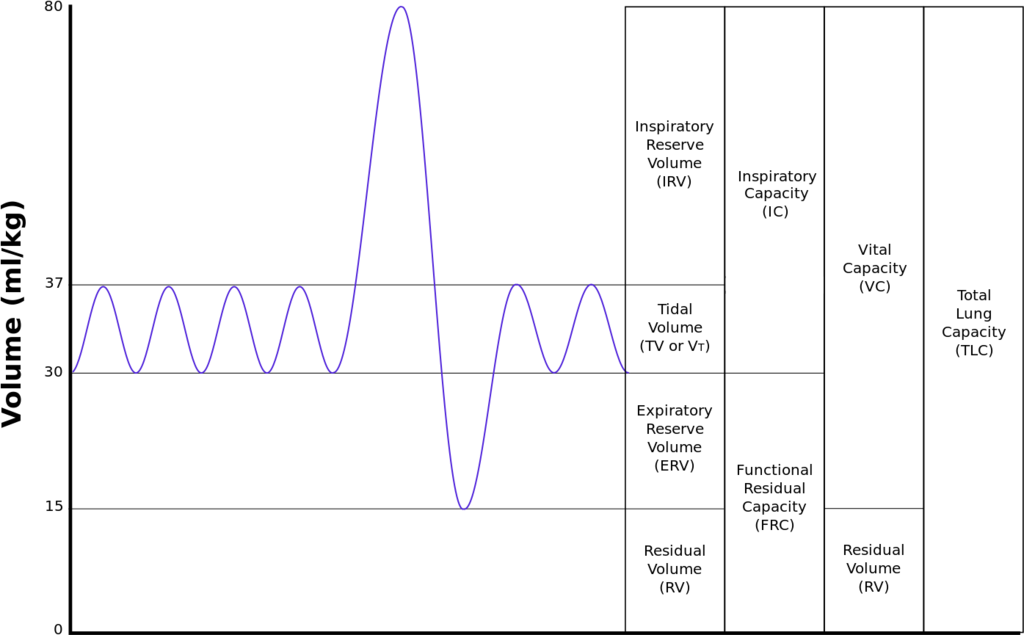
FEV1 – forced expiratory volume in 1s
FVC – forced vital capacity
So back to COPD (there maybe a little more physiology to come, don’t worry)
- Obstructive airway disease. What does that mean in the context of the physiology above?
o To reach a FEV1/FVC ratio of <0.7, you would either need to reduce the expiratory volume or increase the vital capacity
§ With obstructed airways the FEV1 reduces
§ This happens through chronically inflamed airways causing:
· Increased numbers of goblet cells
· Fibrosis
· Narrowing of small airways
· Airway collapse
· Dilation or destruction of respiratory bronchioles or alveolar
The loss of airways and the increased resistance increases the work of breathing and reduces minute volume (ie the amount of air moved per minute) – which can lead to hypercarbia
Airway destruction and increased diffusion distances through inflammatory changes, hyperplasia and increased mucus secretion leads to V/Q mismatches and hypoxia
These are the historic presentations of emphysema and bronchitis, as well as the description of COPD patients as pink puffers and blue bloaters
The reality and the reason we have moved away from these characteristics is that there is destruction across the board and every patient is a spectrum of V/Q mismatch and airway compromise.
Baring this in mind – it’s worth thinking about of the lung responds to this development of pathology
o Normal physiological response to hypoxia is vasodilation to improve blood flow, and hence oxygenation
o In the lung, oxygenation is dependent on ventilation rather than blood flow, so the opposite effect happens – vasoconstriction rather than dilation to improve the blood supply to well oxygenated areas of lung
§ This gradually leads to increased pulmonary artery pressures as more and more of the lung vasoconstricts as the chronic, progressive effect of COPD takes hold
§ This is the basic pathophysiology behind right ventricular dilation and hypertrophy and cor pulmonale in COPD patients
Concepts worth being aware of and understanding in respiratory physiology:
DEAD SPACE
- Lung that receives ventilation but no blood flow
SHUNT
- Perfused areas of lung that receive no ventilation
COPD presentations to ED
So a lot of our COPD presentation are due to exacerbations – that is deviations from the patients baseline respiratory functioning.
Whilst COPD is a chronic condition characterized by a lack of reversibility, something has triggered it to be suddenly worse, and this is the premise we work from.
What causes exacerbations?
Most are infective
o Viral (Rhinovirus, Influenza, COVID, Adenovirus, Para-influenza, RSV)
o Bacterial (Haemophilus Influenza, Strep Pneumoniae, Moraxella Catarrhalis, Staph Aureus, Pseudomonas Aeurinosa)
Some are pollutants
o Nitrogen Dioxide
o Particulates
o Sulphur Dioxide
o Ozone
But about 30% have an unknown aetiology
The cause has relevance to us a the front door, it gives us one big question. Do they need Antibiotics or not?
So how do we work them up?
- As always in ED we often try to do things simultaneously and we have to think about a broader differential. (Don’t just get drawn into saying its their COPD, people with COPD are often co-morbid and wheeze has other differentials)
So obviously we want a simple, concise history and examination
- Have they got any infective features?
o Do they THINK they have a chest infection (the long term COPDers will often know)
o Have they got a fever, change in their cough?
- Could it be something other than COPD
o Do they have any chest pain (MI leading to heart failure, PE)
o Leg swelling (Cor pulmonale, DVT/PE)
o Is their breathing difficulty positional (effusion)
o Did it start suddenly or gradually (Sudden more likely to be PE/MI/pneumothorax)
- What’s their breathing like? Often the pattern and quality of their breathing is more important then how their chest sounds. They are likely to have poor sounding chests a lot of the time and we cant fix them past what their baseline is
o Can they complete sentences
o Is it laboured
o Are they tripoding and using accessory muscles
- We need to consider non chest causes for their change in breathing patterns
o Metabolic Disturbances
o Intraabdominal Pathology
- Baseline functioning and social circumstances
o Establishing their baseline exercise tolerance (ET), in metres or yards is extremely important
§ Firstly it gives an idea of what level of respiratory function we are aiming to restore
§ Secondly, escalation planning, COPDers almost never get intubated and ventilated. For most their ceiling of care will be NIV and their ET is a strong indicator of their functioning
o We should always be trying to plan for deteriorations and having the information at hand
o Part of the NIV checklist is making sure they have an escalation status
- We also want to know other ways that their chest is bad
o We’ve mention ET
o Home O2
o Nebs at home
o NIV at home
o Prior hospital NIV use
All of these would be a marker of someone with a worse baseline and higher likelihood of respiratory failure and being more sick during their hospital stay
Our intial investigations are important and can provide us with almost instant feedback
1. VBG – the initial gas on arrival/assessment will give us their current gas exchange and is as useful as an ABG. 90% of the time our initial management will be medical to try and optimize their ventilation
In the absence of profound hypoxia a GOOD sats trace is as good as an ABG – there is a gradual move away from ABGs in all COPD patients all of the time
2. CXR – vital for both forward planning and ruling out pneumothorax (incase they need NIV) as well as looking for causes of the exacerbation ie pneumonia or effusions
a. Always double check that what you think might be a pneumothorax isn’t a large bullae by looking at old CXRs, we don’t want to be putting drains in bullae, that doesn’t end well
Both VBGs and CXR can be organized to happen within 5-10mins of arrival into resus in an unwell patient
3. Bloods – they take a little longer but looking for signs of occult infection can be helpful and the role of PCT can be vital
4. Consider cultures – especially sputum if the patient is having recurrent exacerbations and frequent abx use, for blood cultures we don’t have to have a pyrexial patient and if we are giving abx it maybe worth considering them
Initial management
Lets consider our treatment options
Reassurance – the element of stress and anxiety can not be underestimated. Imagine not being able to breathe. All the time. Whenever you do anything. Just think about how unsettling it can be to just have a blocked nose.
Positioning – patients will often find their own position that is optimal for their ventilation. We should be helping them find and maintain this, however they are most comfortable
Oxygen – its simple, hypoxia kills. However, its not at all simple and needs further discussion
Bronchodilators
- Beta agonist – salbutamol (we should know dosing and side effects well). There is evidence that 2.5mg is as effective as 5mg but ive not looked into the papers to see the context of this
- Anticholinergic – ipratropium, 500micrograms (can be given 3 times)
Steroids – every patient should be receive steroids, either pred or hydrocort.
- The criteria is anyone who has symptoms that are intervening with their day to day life. My argument is that if they’ve come to ED then their day to day life has been interfered with
- Oral as good as IV, with perfect bioavailability, so if they can take and absorb then give it oral
- 30-40mg of pred for 5/7 is a reasonable prescription if you are thinking about discharging
- 5mg of pred is roughly equivalent to 20mg of hydrocortisone
Magnesium – Intravenous
- https://pubmed.ncbi.nlm.nih.gov/35616126/
- No significant evidence that it makes any difference, similar to asthma, it maybe beneficial or it may not
- But tends to be safe
Zero evidence for nebulized magnesium
Respiratory Support
- NIV,
o COPD patients needing NIV have a mortality rate of 25-30%
- Intubation and ventilation
Adrenaline
Aminophylline – should only be used if other treatments aren’t sufficient and there is no evidence of improvement beyond nebulisers
- Unlike magnesium It has a higher risk profile of side effects and the reality is that they probably need NIV
Doxapram
Now lets start with Oxygen and hypoxic drive
Passive oxygenation – this is the concept of entraining oxygen due to the diffusion gradient from an O2 mask through to the capillaries.
- As the O2 diffuses into the blood and is whisked away, this creates and maintains the gradient throughout the bronchial tree to the mask
- If you passively oxygenate a non breathing person it can be 5-10 minutes before they desaturate due to this entraining of O2
o Obviously this works with high concentration of O2
Now hypoxic drive
- Now an outdated concept
- Too much O2 can leave to hypercapnic respiratory failure
- Patients with chronic hypoxia are at risk and more the hypoxia the more profound the response to O2
- Hypercapnic respiratory failure with O2 is probably from a worsening of the V/Q mismatch and the Haldane effect
o In chronic lung disease the hypoxic areas of the lung have vasoconstriction to reduce shunt
§ These are often poorly ventilated areas
o The addition of higher concentration O2 reverses this process, causing vasodilation in these previously constricted areas.
§ Reduces the perfusion of the better ventilated areas
§ Therefore leading to worsening V/Q mismatch
o The Haldane effect
§ Deoxygenated haemoglobin binds to CO2 with much greater affinity when compared to oxygenated haemoglobin
§ A lot of COPDers will have some element of deoxyhaemaglobin in circulation
§ When you give them O2 the deoxyhaemoglobin will release CO2 to bind with O2, releasing the CO2 in the blood where it is metabolically active
§ These COPD patients are unable to increase their minute ventilation to clear the CO2 and so the level in the blood rises
- BTS guidelines suggest that anyone with concerns for hypercapnic respiratory failure should have target sats of 88-92% whilst pending blood gas results – this includes COPD, CF, chronically obese patient at risk of obesity hypoventilation and those with chest wall deformity. Outside of these concerns normal saturations should be targeted
- There is RTC evidence to back up the concept of targeted sats in COPD and that this patient group has better outcomes
o https://pubmed.ncbi.nlm.nih.gov/33243839/
o https://pubmed.ncbi.nlm.nih.gov/25979080/
o https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2957540/
These studies suggest that we should be targeting lower sat ranges in most if not all of our COPD patients, but definitely until their blood gases show us differently
So with this in mind we should be making sure we are delivering O2 in a controlled manner – that is with a venturi mask – so we know exactly what % O2 they are receiving, allowing us to safely and effectively titrate to the patients sats
NIV
The aim is to try and bridge them through their acute exacerbation
Has a significant associated mortality – if they need NIV for their T2Rf they are demonstrating how frail their respiratory physiology is
- With this in mind we should be doing everything we can to optimize them medically and making escalation plans before starting NIV – what are we going to do if it doesn’t work?
- COPD patients in T2RF are extremely difficult to wean from I+V and often end up being unable to do so. They have a mortality of >40%
- The leeds criteria is for COPD patients with respiratory acidosis
How Does it work?
- Improving laminar flow by splinting airways and alveoli, reducing atelectasis, improving compliance and overall reducing the work of breathing
- Increase in intrathoracic pressure reduces the afterload reducing the work of the LV
Indications
Respiratory failure refractory to simple measures
Ie T2RF in COPD persistent after medical management, T1RF in COVID requiring more than 40% FiO2, T1RF in heart failure and pulmonary oedema
Contraindications
- Reduced GCS, if not maintaining own airway then likely to inflate their stomach causing vomiting and aspiration
- Facial injury/prior surgery precluding mask fitting
- Need for urgent intubation
- Vomiting
- Haemodyamic compromise (PEEP causes reduced preload in the short term causing hypotension)
- Uncooperative patient
- Pneumothorax without a drain (can tension with added respiratory pressure)
How to set up NIV
1. Whats the indication? Does an ED senior know about the patient
2. Any contraindications?
3. Have they had a CXR?
4. Is the BP good enough?
5. Does the patient have an escalation plan? Make a plan as NIV is started, what happens if NIV fails?
6. Fit the mask
7. Set up the intial pressures
a. PEEP of 5 and IPAP of 10/12 is reasonable starting place for someone who is NIV naive
i. The higher the PEEP the more likely haemodynamic compromise is, unlikely with PEEP below 8cmH2O but starting a fluid bolus with the NIV can prevent initial problems
Adjusting NIV
Targets for CPAP
- Improve oxygenation without compromises in cardiac output
- Gradual increase in PEEP to allow for reduction in FiO2
o NOT causing haemodynamic compromise or barotrauma
- Typically wean up from 5cmH2O to 15 over a prolonged period, reducing FiO2 as compliance and shunt improves, can go to 20cmH2O
o Limited by patient comfort and haemodyamics
Targets for NIV
- Typical starting pressures of 10-12 inspiratory pressure (IPAP) and 5 of expiratory pressure (EPAP)
o Patients with prior BiPAP use may tolerate intial higher pressures
- Dynamic adjustment titrating to CO2, O2 and haemodynamics
o EPAP will improve O2
o The difference between inspired and expired pressures will improve minute volume and so eliminate CO2
- Can wean to a maximum of 25cmH2O in any mode of ventilation
Mask fit and leak maybe the limiting factors of how effective the NIV is
Potenial complications
Pressure related
- Gastric insufflation
- Pneumothorax
Airflow
- Dry airways
- Nasal congestion
Major complicaitons
- Aspiration
- Hypotension
- Mucous plugging
Mask related
- Claustrophobia
- Air leak
- Pressure sore
A little more respiratory physiology:
As we have already discussed, the V/Q mismatch in COPD leads to a lot of the physiological problems we encounter. And our treatments, ie high oxygen concentrations, are likely to worsen the mismatch rather than help it.
Whilst thinking about V/Q mismatch, dead space and shunt we can start to think even more about the way the lung functions and what effect gravity and positioning can have on the lung and V/Q mismatch. The model for this is West’s Zones of the Lung and a dumbed down, one sentence explanation would be in well, spontaneously breathing individuals the effect of gravity on pulmonary pressures creates a difference in perfusion from the top of the lung (less blood supply) to the bottom of the lung (more blood supply). This can be expressed by breaking the lungs down into different zones reflecting the different pressures in those areas. There are 4 Zones. Try not to think about these as anatomical zones, the model relating to gravity only works in spontaneously breathing, non ventilated patients where the atmospheric pressure is constant. These are more physiological zones. Each zone is defined as the relative pressures in that area. It may help to think of the thorax as a closed box, similar to the way we think about cerebral perfusion - where a balance of ICP and BP are needed to facilitate the perfusion of the brain.
Zone 1: Alveolar pressure > Pulmonary Artery Pressure > Pulmonary Capillary Pressure
In this part of the lung, the “air pressure” in the alveolar is high enough to stop that area of lung from being perfused - essentially creating dead space. This happens almost universally in ventilated patients - increasing the “air pressure” in the thorax. It can also happen in anyone who has a low pulmonary arterial pressure. Think about hypovolaemia (ie major haemorrhage) or RV dysfunction.
Its worth thinking about this Zone in our COPD patients that we are starting NIV on - we are adding PEEP onto unwell, often intra-vascular deplete patients (they are often dry from increased insensible losses +/- infective/sepsis elements). If we put this together - the increased alveolar pressure from the PEEP and reduced RV preload from being dry is likely to cause more Zone 1 in the lung ie dead space - something we really want to avoid - giving them some IV fluids in the lead up and as we start NIV may go some way to alleviate this with improving RV preload.
Zone 2: Alveolar Pressure < Pulmonary Artery Pressure > Pulmonary Capillary/venous Pressure =/< alveolar pressure
Zone 2 represents the areas of the lung where the peaks of Pulmonary Systole causes perfusion but the MAP is equal to the alveolar pressure, so there is only transient blood flow. The official definition is “Zone 2 is that part of the lung between the levels at which arterial and alveolar pressure are equal and venous and alveolar pressure are equal”
This Zone probably represents most of the lung in most healthy people in most postures and when we are supporting ventilation we should be thinking about optimizing as much of the lung to this zone.
Zone 3: Pulmonary Artery Pressure > Venous > Alveolar Pressure
This Zone represents areas of the lung that are consistently perfused but may also represent areas of poor ventilation and atelectasis that may contain no air. Potentially areas of shunt.
Zone 4: Pulmonary Artery Pressure > Pulmonary Interstitial Pressure > Pulmonary Venous Pressure > Alveolar Pressure
This Zone isnt always recognised but happens when there is significant atelectasis or oedema causing the interstitial fluid pressure to be greater than the venous pressure. This means that the blood flow is dependent on the relationship between the Arterial Pressure and the Interstitial Pressure
So when thinking about these different physiological areas of lung and the relationships between perfusion and ventilation we should consider how they reflect our clinical scenarios and the application of positive airway pressure - both in intubated patients but also in our NIV patients (which is how we got to this point with COPD)
As we have already mentioned, limiting the amount of PEEP and making sure we maximize Right Sided Cardiac Output is going to help the V/Q mismatch in our COPD patients that we start on NIV. Reducing the amount of lung that is in Zone 1
Obviously there is a balance to be struck, and we need to give enough PEEP to improve compliance and reduce the work of breathing as well as recruiting (opening up) enough lung to improve their ventilation and oxygenation
There is also a diminished effect in NIV patients compared to intubated and ventilated patients due to the leak and lack of closed circuit, but we can still think about the principles
The other interventions we can do is to try and reduce the amount of lung in zone 4
If they are wet, or consolidated, or in a bad position reducing their ventilation and increasing the interstitial pressure.
Chest physio/good posture +/- furosemide for oedema may help improve the situation.
Obviously this is all very situation dependent